Cleanliness and Corrosion Mitigation
Any metal that comes into contact with the electrolyte could corrode.
Several types of corrosion commonly occur – and in several ways.
A critical factor in preventing corrosion in electronics is maintaining the state of cleanliness. This is not easy. Corrosion is defined as the deterioration of a material or its properties due to a reaction of that material with its chemical environment.1 So, to prevent corrosion from occurring, either the material or the chemical environment must be adjusted. Adjusting the material usually means replacing it with a less reactive material or applying a protective coating. Adjusting the chemical environment usually means removing ionic species through cleaning, and removing moisture, usually with a conformal coating or hermetic package. Ionic species and moisture are problematic because they form an electrolyte able to conduct ions and electricity. Any metal that comes into contact with the electrolyte can begin to corrode.
Several types of corrosion can commonly occur on electronics assemblies.
Gas phase corrosion. Some metals used in electronics, such as copper, nickel and silver, are susceptible to gas phase corrosion. In the cases of copper and nickel, the metals react with oxygen in the air to form a thin oxide layer and an unsolderable surface. This is why surface finishes are used. They serve as protective coatings by preventing copper from oxidizing and retaining a solderable pad on a bare board. One such surface finish, immersion silver, protects the underlying copper, but the silver itself is susceptible to attack from sulfur-containing materials and gases in the atmosphere, leading to tarnish (Figure 1). Prevention of exposure to sources of sulfur is key to preventing tarnish from occurring. Sulfur is found in air pollution, rubber bands, latex gloves, desiccant, and sulfur bearing paper used to separate parts.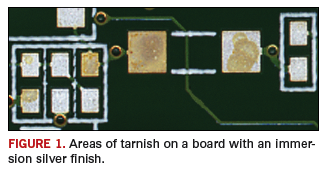
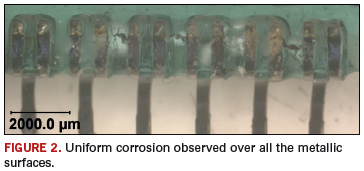
Uniform corrosion. Uniform corrosion is evenly distributed across the surface with the rate of corrosion being the same over the entire surface (Figure 2). One way to determine the severity of the corrosion is to measure the thickness or penetration of the corrosion product. Uniform corrosion is dependent on the material’s composition and its environment. The result is a thinning of the material until failure occurs.2 Uniform corrosion can be mitigated by removing or preventing ionic residues and preventing moisture.
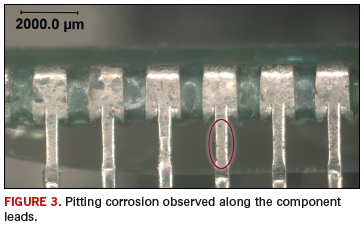
Pitting corrosion. Pitting corrosion is a localized form of corrosion where the bulk material may remain passive, but pits or holes in the metal surface suffer localized and rapid surface degradation (Figure 3). Chloride ions are notorious for forming pitting corrosion, and once a pit is formed, the environmental attack is autocatalytic, meaning the reaction product is itself the catalyst for the reaction.3 Pitting corrosion can be mitigated by removing or preventing ionic residues and preventing moisture.
Electrolytic metal migration. In the presence of moisture and an electric field, electrolytic metal migration occurs when metal ions migrate to a cathodically (negatively) charged surface and form dendrites. The dendrites grow and eventually bridge the gap and create an electrical short. Materials susceptible to metal migration are gold, silver, copper, palladium and lead. These metals have stable ions in aqueous solution that are able to travel from the positive electrode (anode) and deposit on the oppositely charged negative electrode (cathode). Less stable ions, such as those of aluminum, form hydroxides or hydroxyl chlorides in the presence of high humidity and chlorides. An example of a copper dendrite is shown in Figure 4. Electrolytic metal migration can be mitigated by removing or preventing ionic residues and moisture.

Galvanic corrosion. Galvanic corrosion occurs when two dissimilar metals come in contact with one another or are connected through a conductive medium such as an electrolyte. A soldered joint is a composite system where many different materials are connected. Within the joint or between joints and other conductive circuitry, DC circuits can be established that will corrode the most anodic material.4 When ionic species are present, such as flux residues and moisture, an electrolyte can form. The corrosion at the metal forming the anode will accelerate, while the corrosion at the cathode will slow down or stop. In a poorly deposited ENIG surface finish, a porous immersion gold layer exposes the underlying electroless nickel. The large difference in electrochemical potential between the nickel and gold causes corrosion of the nickel layer, while the gold acts as a powerful cathode. As corrosion proceeds, pitting of the nickel can extend into the underlying copper and cause further corrosion. If there is no porosity in the gold layer, but instead, a gap between the metallic component and the resist edge, the metallic layers can be exposed to solution allowing galvanic corrosion.5
Table 1 lists metals in order of their relative activity in sea water (the Galvanic Table from MIL-STD-889, Dissimilar Metals). Generally, the closer the metals are to one another in the listing, the more compatible. However, in any combination of dissimilar metals, the more anodic metal will preferentially corrode. To prevent galvanic corrosion, careful selection of adjacent materials must occur in the design phase. To mitigate galvanic corrosion from occurring in the field, an electrolyte must be prevented from depositing on any connection of dissimilar metals.

Corrosion can be mitigated by preventing electrolytes from forming. This is accomplished by ensuring that any ionic residues are removed after component handling, bare board fabrication, and assembly, as well as preventing salts from depositing on the assembly from extreme environmental conditions. Moisture can be prevented by using a conformal coating or hermetic package. Also, materials selection in the design phase is important so that metals with dissimilar electrochemical potentials are not directly connected. If dissimilar metals must be used, such as when using specific surface finishes, like ENIG, then ensuring good bare board construction is a critical step in reliable, corrosion-free electronics.
References
1. Bob Stump, National Defense Authorization Act for Fiscal Year 2003. Pub. L.107-314. 2. Stat. 116.2658, December 2002.
2. B. D. Craig, R. A. Lane and D. H. Rose, “Corrosion Prevention and Control: A Program Management Guide for Selecting Materials,” AMMTIAC, 2006: 61.
3. Electronic Device Failure Analysis Society, Microelectronics Failure Analysis: Desk Reference, ASM International, 2004: 3.2.
4. Perry L. Martin, Electronic Failure Analysis Handbook, McGraw-Hill, 1999: 13.42.
5. Rajan Ambat, “A Review of Corrosion and Environmental Effects on Electronics,” 2006.
ACI Technologies Inc. (aciusa.org) is the National Center of Excellence in Electronics Manufacturing, specializing in manufacturing services, IPC standards and manufacturing training, failure analysis and other analytical services. This column appears monthly.




