Under-Component Cleaning: How Low Can You Go?
Why visual indicators as a cleanliness gauge may be dangerous practice.
Preventing field failures due to electrochemical migration used to be relatively easy: Use a water-soluble flux and clean the board after soldering. This solution no longer holds true, and sometimes causes more problems than it prevents.
Water-clean fluxes, also known as organic acid (OA) fluxes, are often preferred over no-clean products for high-reliability applications because of their higher activity. They are more effective at removing oxides, promoting wetting and overcoming solderability variations. Problems arise when their residues are not fully cleaned from the assembly. OA fluxes are active at room temperature, and all they need to begin damaging the assembly is DC voltage and atmospheric moisture. When OA flux residues remain on a circuit assembly, the concern is not a matter of if the circuit board will fail; it is a matter of when it will fail.
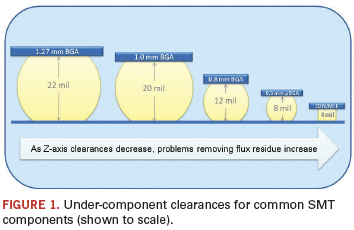
Fully cleaning the harmful residues gets increasingly difficult as components get smaller and standoff heights shrink (Figure 1). Many low-standoff components are designed only for no-clean soldering processes; however, OEMs and assemblers sometimes have no choice but to use them in water-clean applications. And without a definitive, nondestructive test for cleanliness, both parties harbor concerns about the long-term reliability of the end-product.
To characterize the cleanability of modern OA solder pastes, a simple experiment was devised. PWBs were assembled with 12 of the industry’s most popular pastes, reflowed in an air atmosphere, cleaned, and visually graded for residues.
Areas easily reached by the wash process, like gullwing or J-lead components, were represented by unpopulated component pads printed with solder paste. Areas obscured by low-standoff components like µBGAs or QFNs were conservatively represented by 0603 components (Figure 2). Reflow was performed in an ERSA Hotflow 3/20, 10-zone reflow oven using typical thermal profiles for SnPb and Pb-free (SAC 305) alloys, and cleaning was performed in a Speedline Aquastorm AS200 inline cleaner at a belt speed of 2 ft./min. Water temperatures of 120°, 140° and 150°F were used. Three boards were processed at each setting; the three scores were averaged and reported.

The gold standard for assessing cleanliness is ion chromatography (IC), but performing IC analysis on a large sample size is time-consuming and cumbersome. To rapidly assess flux residue cleanability, a visual cleanliness scale
(Table 1) was developed. It should be noted that visual cleanliness does not necessarily indicate ionic cleanliness, but visible residues generally do indicate residual ionic contamination. Therefore, the only grade on this scale that could be considered a “pass” is a 5. Several areas assigned a score of 5 were confirmed clean with ion chromatography. Scores of 4 or more indicate that the reflow and/or cleaning process could likely be dialed in to produce acceptable results. Scores of 3, 2 or 1 are unacceptable process failures.

Test Results
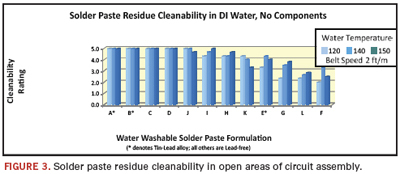
The first test assessed the overall process friendliness of the solder pastes by evaluating their cleanability on unobstructed pads using only deionized water. Results (Figure 3) are ranked in order of their cleanability. Of the 12 pastes tested:
- Five demonstrated easy cleanability.
- Two posted scores in the 4+ range, indicating potentially good cleanability.
- Five pastes failed.
- Two of the failures showed exceptionally poor cleanability.
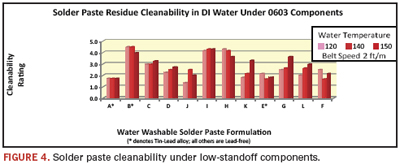
The next test mechanically removed the 0603s and examined the post-cleaning residue underneath. Figure 4 presents them in the same order as in Figure 3.
Under-component cleaning results differ substantially from the bare board results:
- Paste A, which demonstrated best-in-class cleanability in visible areas, actually showed the worst results in obstructed areas.
- All the pastes left some degree of residue.
- Three pastes scored 4s at some, but not all, temperatures.
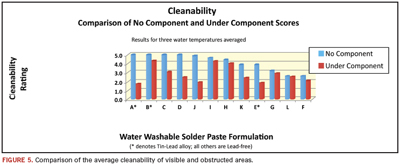
The average cleanliness scores of visible and obstructed areas were then compared (Figure 5). The comparison produced eye-opening results:
- Only one of the five pastes that demonstrated excellent cleanability in the open areas showed good cleanability under the components. The other four received failing grades.
- The two pastes that showed potential for good cleanability on the bare board areas showed similarly good cleanability in the obstructed areas (pastes H and I).
- The three pastes that produced obviously unacceptable results in the visible areas produced similarly unacceptable results in non-visible areas.
- Half the pastes tested showed substantial drops (more than one grade point) in cleanability when the different areas are compared.
The cleaniness differences between the two areas are obvious – what you see is not necessarily what you get. Relying on visual indicators to gauge an assembly’s cleanliness may be dangerous practice, depending on the solder paste formulation and processes to which it has been exposed.
The ionograph test that is commonly used to monitor cleaning processes’ effectiveness can also lull users into a false sense of security. In many cases, the alcohol-water mixture in the test solution does not completely dissolve baked-on residues under the low-standoff components, and the test fails to detect the actual contamination levels. Even if it does fully dissolve the residues and detect all the contamination, the test averages it across the entire surface area of the assembly, diluting the localized dangerous level to a perceived overall “safe” level.
Improving Cleanliness
The next phase of the experiment added a cleaning solution to the wash. Cleaning agents work in two main ways: They lower the surface tension of the water so it can reach into tight areas, and they help act on the residues to dissolve them.
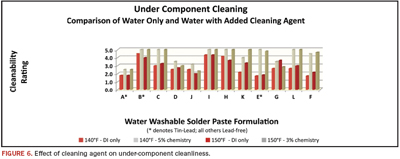
At wash temperatures of 140°F, introduction of a cleaning agent at a 5% concentration dramatically improved under-component cleaning. At 150°F, only a 3% concentration was required to produce similar results (Figure 6). Introduction of cleaning chemistry, even at low concentrations, made a substantial impact on the cleanliness of the areas obscured by the low clearance components:
- In 12 of 24 cases, the introduction of a cleaning agent produced a perfect score of 5. When cleaning agents were not used, no perfect scores were recorded over the course of 72 trials.
- In all cases except one, the addition of chemistry improved cleanliness under the component.
- Pastes L and F, which demonstrated some of the poorest cleanability with DI water alone, scored 5 and 4+ when cleaning agents were added to their wash process.
For many years, OA solder pastes were referred to as “water-soluble.” Recently, that term has migrated to “water-washable” or “water-cleanable,” indicating that modern formulations do not exhibit the easy solubility of their predecessors. The current data support this migration of terminology, demonstrating that residues under components can be satisfactorily washed away – but not with water alone.
Consider trying to wash a car, household laundry or dirty dishes with water only. The car, clothes or dishes would be extremely difficult to clean without the assistance of detergents. Industrial cleaning processes are no different from domestic ones; they all work better when they incorporate cleaning agents. In the case of circuit assemblies and modern solder pastes, cleaning chemistries are rapidly becoming a necessity. For low-standoff components like microBGAs and QFNs, engineered cleaning solutions are assuring ionic cleanliness and alleviating the concerns of electrochemically induced field failures for everyone in the supply chain.
Harald Wack, Ph.D., Umut Tosun, Joachim Becht, Ph.D., and Helmut Schweigart, Ph.D. are with Zestron (zestron.com); hwack@zestron.com. Chrys Shea is founder of Shea Engineering Services (sheaengineering.com).




