Post-Reflow Residue Results Following pH-Neutral Cleaner Application
A study of 40 leaded and Pb-free solders compares alkaline to newer cleaning technologies.
PH-neutral product technologies that are fully compatible with anodized aluminum parts, sensitive metals and other challenging substrates are now available. Despite the absence of alkalinity, pH-neutral chemistries can still complete the job of defluxing agents. Their unique physical properties also come into play as users strive for minimal equipment wear-and-tear, which had not been the case in the past. Equipment replacements are costly and can add significantly to the overall process cost. Safety is also improved if operators can completely avoid working with caustic materials.
The performance of today’s pH-neutral, aqueous cleaning agents hinges on previously unknown reaction pathways, which in turn permit the complete removal of any alkalinity in the product. While prior cleaning pathways relied heavily on solubilization and saponification, novel pathways include but are not limited to dipole mechanisms (Figure 1).
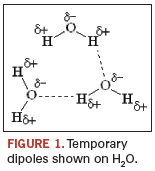
The latter introduces the benefits of:
- High reactivity, especially for cleaning under low-standoff components.
- Highest level of material compatibility known to date.
- Low operating concentrations of 5 to 15%.
- Easy disposal; i.e., no permits or costs due to the neutralization of alkaline products.
- Operator safety.
- Reduced shipping costs due to noncorrosive nature.
For predominantly competitive reasons, experimental data on pH-neutral cleaners remained unpublished in 2009. Research presented in here focuses on internal qualification experiments leading up to the product’s market introduction. Various customer studies completed in 2009 will be presented shortly.
Test Parameters
This study was initiated in 2005 to evaluate new product technologies developed in response to challenges presented by use of standard alkaline cleaning products for defluxing processes. One of the often-cited drawbacks of alkaline materials is their effect on sensitive metals, which include anodized and bare aluminum, copper, different metal alloys, plastics, materials of construction, inks and others. To mitigate but not eliminate any potential surface reaction, chemical companies generally add corrosion inhibitors to alkaline products. These additives, however, pose limitations, as they are chemically altered during regular production conditions, thereby introducing an unwanted variation in effectiveness.
Furthermore, alkalinity introduces a pH-level that requires water treatment in most municipalities. PH-levels above 7 cannot be considered pH neutral by definition and must be actively neutralized since they harm the environment.1 This implies an additional process step for the effluent water, which in turn adds cost to the overall process. PH-neutral products, on the other hand, do not require water neutralization and save overall process costs.
Initially the authors’ objective was to establish that a pH-neutral cleaning agent effectively cleans common flux residues at comparable process settings, as we have grown accustomed to defluxing with pH-alkaline agents. For that reason, 40 frequently used Pb-free and leaded solder pastes from leading global manufacturers were chosen and reflowed according to the manufacturers’ designated profiles. A detailed analysis was performed to assess visual cleanliness under 4 to 60x magnification, according to IPC-A-610.2 A visual inspection of the initial segment was completed first, as supplemental analytical results would verify absolute and quantifiable cleanliness. Test results were independently evaluated by three investigators to mitigate subjectivity. Moreover, ion chromatography data according to IPC-TM-650, method 2.3.283 were used to complement the analytical analysis of this initial study.
A concentration level of 10% and 125°F were chosen as the initial set-points (Table 1). This not only represents the latest industry trend toward lower operating temperatures and concentrations, but also meets the common goal of reducing the amount of organic effluent flowing from the chemical isolation section to the drain. (As an aside, any reduction in cleaning agent concentration, assuming constant cleaning effectiveness, supports cost-cutting efforts.) At first, the cleaning temperature was set at 125ºF, which in turn reduces energy consumption and evaporative losses. As many companies are not in position to invest in advanced vapor recovery systems, the authors felt that these parameters would benefit potential and current users by optimizing their cleaning process. However, all parameters combined naturally challenged the performance of the cleaning agents tested. A secondary objective of this study was to demonstrate the long-term performance potential of the tested cleaning technologies. As new guidelines and legislative policies are being contemplated, companies must be prepared for current and future regulations.

Comparable Results
The chosen solder pastes are a representative sample of currently used products in the industry. They included leaded and Pb-free materials, and the investigators chose individual reflow profiles to best simulate real production conditions. Average cleaning results above 4 were considered clean (Figure 2), based on the presence of experimental error, as well as qualitative subjectivity. [Ed.: Comparisons to alkaline cleaners also were made and will be presented at SMTA International in October 2010.] Also, this assessment was supported by additional ion chromatography data on numerous substrates. The pH-neutral product at 10% concentration achieved results of 4 and above in 34 of 40 pastes. This equates to 85% of all tested cases. Four pastes achieved a rating of 2 or below. Increasing the temperature to 160ºF improved the cleaning results to 39 of 40. This constitutes a remarkable 97.5% of all cases tested. It should be noted that visual cleanliness does not necessarily indicate ionic cleanliness, but visible residues generally do indicate residual ionic contamination. Therefore, the only grade on this scale that could be considered a “pass” is a 5. Several areas assigned a score of 5 were confirmed clean with ion chromatography. Scores of 4 or more indicate that the reflow or cleaning process could likely be dialed in to produce acceptable results. Scores of 3, 2 or 1 are unacceptable process failures. Figures 2 and 3 show the cleaning results for leaded and Pb-free solder pastes.
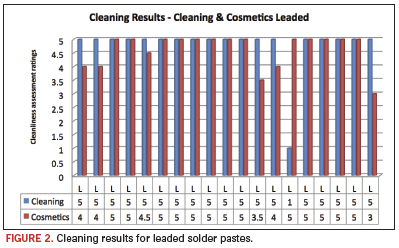
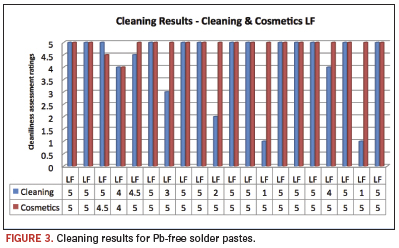
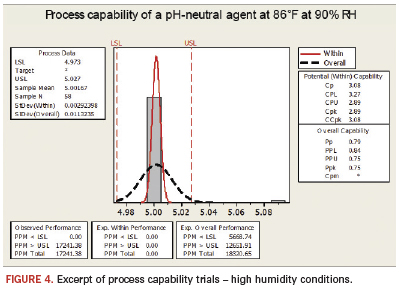
These findings are significant, as for the first time pH-neutral agents have demonstrated cleanliness levels equivalent to those of pH-alkaline cleaning technologies. PH-neutral cleaning agents do, however, add a new level of material compatibility and ease of effluent treatment to the equation. These results truly showcase the future of aqueous defluxing chemistry. Figure 4 shows results of a recent evaluation of the pH-neutral cleaning agent in a high-end inline cleaning process at a provider of comprehensive electronics design and production. Conditions were set at 86°F/30°C and 90% RH in a test chamber. The figure shows the defined process limits, as well as the range of results that passed the test. Eighty-eight boards were tested under the same high humidity conditions. One board failed the high humidity conditions, as the assessed readings did not deliver values according to the customer’s specification.
Flux residues and impurities under low-standoff components are often difficult to remove, since not all water-based cleaning agents are designed to enter this minimal gap between package and board. Therefore, cleaning agent entrapment becomes a common issue: The cleaning agent is not properly rinsed away after application, and impurities together with cleaning agent residues are trapped under low-standoff components or around solder joints, making PCB boards more vulnerable to creeping and long-term corrosion. This, in turn, can cause failure, including field failures. Studies are underway to further examine dissolved flux residues and their respective effect on contributing to corrosion. Generally, it is commonly understood that pH-neutral defluxing technologies can limit these risks of corrosion underneath components, while providing higher levels of material compatibility.
Acknowledgments
Zestron thanks Ersa for its generous support with the Hotflow reflow oven, and Speedline Technologies for its generous support with the Aquastorm AS 200 cleaner.
References
1. F.R. Cala and A.E Winston, Handbook of Aqueous Cleaning Technology for Electronic Assemblies, Electrochemical Publications, November 2005, pp. 64-65.
2. IPC-A-610E, Acceptability of Electronic Assemblies, April 2010. http://www.ipc.org/4.0_Knowledge/4.1_Standards/revstat1.htm
3. IPC-TM-650, method 2.3.28, Ion Chromatography - Dionex ICS-1100, http://www.ipc.org/4.0_Knowledge/4.1_Standards/test/2.3.28A.pdf.
Bibliography
1. Chrys Shea, “Under Component Cleaning – How Low Can You Go?” CIRCUITS ASSEMBLY, July 2010.
2. Zestron Europe, “Reliable Coating with Proper Cleaning - Cleaning Process Benefits for EADS.” Process Cleaning Magazine, January 2009.
3. Dr. Harald Wack, “The Future of Cleaning OA fluxes,” SMT, September 2009.
4. Zestron in cooperation with AAT, “Thermal Residue Fingerprinting: A Revolutionary Approach to Develop a Selective Cleaning Solution,” APEX 2009.
5. Zestron, “Fluid Flow Mechanics: Key to Low Standoff Cleaning,” IPC Midwest, 2008.
6. Dr. Harald Wack, “Eutectic and Lead-Free Defluxing in One Single Process,” SMT, March 2007.
7. Umut Tosun, “Cleaning Lead-Free Prior to Conformal Coating? Risks and Implications,” IPC Apex, February 2006.
8. Andreas Muehlbauer, Ph.D., “Are Lead-Free Assemblies Specifically Endangered by Climatic Safety?” IPC Apex, February 2004.
Harald Wack, Ph.D., is president; Joachim Becht, Ph.D., is head of R&D; Michael McCutchen is sales and marketing manager; and Umut Tosun is application technology manager of Zestron America (Zestron.com); h.wack@zestronusa.com.




