2005 Articles
MEMS in Medicine
These sense-mimicking chips may herald a new age of bionics.
MEMS, or microelectromechanical systems, are tiny chips that can be produced by semiconductor processes to combine mechanical sensing, control and motion to solid-state electronics to deliver extraordinary functionality and versatility. Essentially all the mechanical and electromechanical machines from the macro-world can now be crafted into a tiny chip that can enter the micro-world and interact with life systems. MEMS can sense pressure, detect motion, measure forces, identify bio-agents, pump and control fluids, and perform other actions that have great value to the medical and biological fields. BioMEMS describes the chips designed specifically for these applications. BioMEMS chips can serve as chemical and biological analysers, micro-pumps and controllers and hearing aid components, among other applications. But in the future, BioMEMS “medical practitioners” could become permanent inhabitants of the body.
MEMS is the ultimate enabling technology for the integration of almost any physical, chemical and biological phenomena that include motion, light, sound, chemistry, biochemistry, radio waves and computation, but all on a single chip. These chips can mimic all our senses and thus eventually be used as body replacements or enhancements heralding a new age of bionics. While simpler systems, such as hearing aids, have been implemented, extraordinary bio-systems will emerge in the future. MEMS will add the vision, noise, tactile feel and other sensory input while the on-chip electronic logic function will serve as the “brain” to condition signals, organize data and control, analyze and integrate input and output. The merging of motion, sensing and computation represents a significant new level in technology.
MEMS devices can possess high-level integration for numerous functions. This unification of functionality encompasses high precision micro-mechanical motion, optics, micro-fluidics, living cell interaction, sound, radio waves and much more. Senses could even be enhanced, making it possible to see in the dark or extend the hearing range. The additional features of computation and centralized control of all actions into a fully integrated system could deliver surprisingly versatile new products. MEMS is also about technology convergence where miniature devices – including gears, propellers, turbines, pumps, mirrors, motors, radio elements, radiation sensors and many types of detectors and other assemblages – are synergistically united in a new micro- and even nano-world. MEMS can combine and integrate complete systems, from a library of parts that includes micron-sized motors, tweezers, pumps, separators, injectors, needles and scalpels. MEMS places systems together on a fully integrated and self-contained single piece of silicon, or other construction material, that previously could only exist in the macroworld. These previously isolated technologies are now converging onto a tiny single chip of silicon.
BioMEMS devices, however, will become the most important class in the future. They can already detect specific ions and molecules, sense pressure within an artery and even help detect defective DNA. MEMS adds the eyes, nose, ears and some senses that humans don’t even possess, but merges mechanics with the electronic brain of semiconductor logic on a single chip. MEMS can also exert control using untiring electrically-powered “muscles” to move, manipulate or pump fluids and dispense drugs. The merging of motion, sensing and computation represents the most advanced level in atechnology that is still embryonic. While much of traditional commodity electronics is leaving America’s shores, the advanced biomedical field remains in good health and growing.
How MEMS Works
MEMS chips can be mass-produced using modified semiconductor processes. Silicon is the most common material and permits transistors and integrated circuits to be formed on the same chip as the mechanical parts. MEMS fabrication involves forming sacrificial and permanent regions within the substrate by processes common to semiconductor manufacturing. Final removal, or release of sacrificial material that can be silicon dioxide (SiO2) creates the 3-D permanent structure that can have moving parts including levers, hinges, gears and even sprocket/drive chain assemblies. Mechanical motion is produced by actuators fabricated during the structure-building processes. Actuators include classes where movement is produced by electrostatic, magnetic, thermal action and other less common means. Some actuators can even derive power from the body. The energy source is typically electricity that can be converted to thermal, photonic or mechanical energy. This ability to produce controlled motion from energy input can provide microscopic pumps, tweezers, abraders, heaters, injectors, reactors and almost any machine that can be imagined. But electro-mechanics also produces extremely small and sensitive devices for detecting and measuring motion, acceleration, pressure, weak electrical signals, ions and specific biological agents – all useful for medical applications.
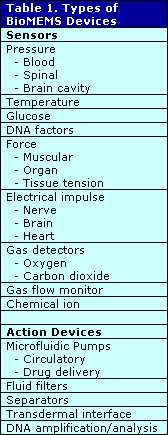 |
We can divide BioMEMS into several categories. The simplest demarcation is to look at the basic operation of the chip. The BioMEMS device may be designed for sensing that can include measuring, monitoring and detecting inside the body or at the surface. Sensing is the largest category in the non-medical MEMS sector and the same probably holds true for medical. The remaining devices can probably be classified as action-type devices whose primary function is to act upon the body and its materials such as fluids, or upon external agents, especially drugs to be administered. In some crossover areas, a single chip fits both categories, such as a device that monitors glucose levels and administers insulin, but this is not very common at this early stage of BioMEMS. The sensors and action categories can be subdivided by function, such as pressure sensor, and then add another subdivision of the function by specific application. Using this approach, a BioMEMS chip inserted into an artery might be classified as sensor, pressure or blood, for example. Table 1 classifies BioMEMS devices.
The MEMS-assisted medical area is relatively new, but substantial work is in progress at universities, government laboratories, hospitals and private industry. The term “BioMEMS” will trigger at least 40,000 hits in an Internet search. We are only at the beginning of this remarkable area of medicine that already has some successes, but holds immeasurable promise. While much work is underway, the inevitable and well-known long path from lab to patient use extends the timeline more so than any other field. The newness and strangeness of MEMS will likely make regulatory approval even more challenging. Science fiction writers have made matters worse by creating MEMS and nanotech phobias.
Sensors; blood pressure. MEMS pressure sensors have been developed and commercialized outside of the medical field and represent one of the largest classes of commercial MEMS products. Their primary use is in the automotive industry where the pressure of fluids and gases must be measured and monitored. Car air conditioners, for example, may use two or three pressure sensors for safety and optimum performance. While environments are completely different, there are similarities between the human body and a vehicle in terms of circulating fluids and critical pressures, so it is no wonder that BioMEMS pressure sensors are an important category. The human circulatory system not only generates pressure within, it has a complex and constantly varying value by the very nature of the heart pump and changes that constantly occur in human “plumbing.” When we add extreme miniaturization and biocompatibility to this already complex system, the BioMEMS challenge is much greater than for any other MEMS area.
Who are some of the players? Integrated Sensing Systems Inc., or ISSYS, performed studies on its own BioMEMS wireless, batteryless, implantable pressure sensor as described by Nader Najafi, Ph.D., president and CEO. Animal studies showed the accuracy and proved the functional feasibility of BioMEMS technology for biological sensing. The results were very encouraging and exceeded the requirements, including high sampling speed and resolution. A second generation of wireless sensors is being developed to provide a total system solution. The company presently offers the two sensors shown in Figure 1 for medical applications and claims to have very high biocompatibility and corrosion resistance. Figure 2 shows the very small feature size of the company’s pressure sensors designed for medical applications. ISSYS is developing intelligent BioMEMS sensors and systems to enhance the quality of medical treatment that can apply to:
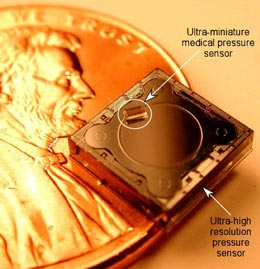 FIGURE 1: Two sensors on a penny; the larger is the world’s most sensitive capacitative pressure sensor and the smaller can be inserted in a diagnostic catheter. (Source: ISSYS, Ypsilanti, MI)
FIGURE 1: Two sensors on a penny; the larger is the world’s most sensitive capacitative pressure sensor and the smaller can be inserted in a diagnostic catheter. (Source: ISSYS, Ypsilanti, MI) |
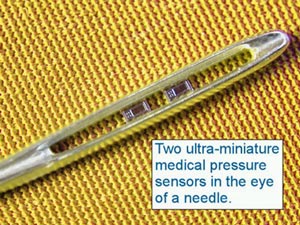 FIGURE 2: Two miniature medical pressure sensors fit in eye of needle. (Source: ISSYS)
FIGURE 2: Two miniature medical pressure sensors fit in eye of needle. (Source: ISSYS) |
- Measure pressure gradients across heart valves to help assess valve disease.
- Diagnose and monitor congestive heart failure.
- Measure cardiac output and compliance.
- Monitor intracranial pressures in hydrocephalus patients.
- Understand glaucoma disease progression and improve patient care.
- Improve gastrointestinal tract diagnostic capabilities to help treat gastro esophageal reflux disease (GERD).
- Assist in diagnosis of urological disorders.
- Measure drug delivery rate for infusion systems.
The Cleveland Clinic Foundation BioMEMS Laboratory is developing systems for biomedical applications since BioMEMS will bring smaller, more accurate, less invasive and more cost-effective biomedical devices. A miniature implantable, wireless BioMEMS pressure sensor is able to monitor physiological measurements, such as aortic aneurysm pressure, and intraocular, intracranial and intervertebral pressures. The BioMEMS Laboratory, which occupies space in the Lerner Research Institute, is a state-of-the-art facility optimized for BioMEMS research and development. This laboratory is equipped with extensive hardware and software tools for design, packaging and characterization of biomedical microsystems. BioMEMS devices and a specially instrumented probe station are available for microactuator characterization, micro-sensor testing and calibration and testing of microfluidic devices to accommodate unique testing requirements. Software packages are used for the layout and performance modeling of BioMEMS device designs. Figure 3 shows some of the implant concept.
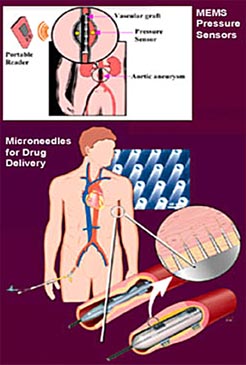 FIGURE 3: MEMS pressure sensor and drug delivery concepts.
FIGURE 3: MEMS pressure sensor and drug delivery concepts. |
Analytical Systems
DNA amplification/analysis/sequencing. MetriGenix started working with Infineon several years ago to develop BioMEMS chips that use DNA chemistry that is more efficient and faster than existing technology. Today’s DNA analysis chips use tiny, shallow wells to contain the DNA and reagents. The MetriGenix design uses a capillary flow-through technique whose prime advantage is to speed up the reactions. Infineon has now embarked on other BioMEMS development projects such as an electronic cell-culture system that gives biological researchers the ability to grow neurons on an electronic substrate and record their electronic behavior.
Minimizing the sample size to use only microliters enables the system to run a battery of tests with significant time saving. Sequencing a sample of DNA to approximately 600 bases using the classic slab gel method takes about four to six hours. The BioMEMS method using a capillary system provides results with the same accuracy and precision in about two or three hours. In the future, they expect to get the same accuracy and precision in 20 to 30 minutes with an optimized version.
ST Microelectronics is also active in BioMEMS. Its prototype lab-on-a-chip is very compact, making it suitable for point-of-care applications (Figure 4). This large and diversified semiconductor company is intensifying its involvement in medical BioMEMS and is seeking partners to market its lab-on-a-chip products. ST has produced MEMS for autos and industry for many years, but this product is its first venture into BioMEMS. It has invested in state-of-the-art research facilities to build up the BioMEMS product lines rather than use retired fabs, as some others have done. Its silicon lab-on-a-chip will become economical through large volume production using the company’s massive semiconductor fabs. This gives BioMEMS products the production benefits usually only found in the huge cleanrooms of semiconductor manufacturing of standard electronics. Instead of the small volumes produced by workshop-style startup companies, BioMEMS will become part of a giant production line. Its strategy is to select products that are marketed through selected partners who have good control over their end markets. The high-volume capacity of ST can bring the niche BioMEMS market into one of high-volume productivity, lower costs and constant market pressure on prices.
 FIGURE 4: ST-Microelectronics’ lab-on-a-chip.
FIGURE 4: ST-Microelectronics’ lab-on-a-chip. |
Lab-on-a-chip permits small amounts of bodily fluids to be tested in seconds. All chemical reactions occur inside the biochip’s buried channels or on its surface. And because the cartridge that carries the chip is self-contained and disposable, the system strongly reduces the cross-contamination risks of conventional multi-step protocols. The ST prototype is designed to handle microfluidics and some of the knowledge is based on expertise in MEMS inkjet printer chips. The prototype integrates two fundamental steps of genetic analysis: amplification and detection. This microscale approach results in time and cost savings. Blood samples typically do not contain enough DNA for analysis and must be amplified, or copied, many times to increase sample size. This is done through a process called polymerase chain reaction (PCR), a technique for replicating or cloning DNA. BioMEMS PCR amplification can be performed in 15 minutes, a process that might ordinarily take one or two days.
A researcher mixes a DNA sample with a polymerase enzyme and DNA primers and passes it through a bank of 12 microscopic channels, each measuring 150 to 200 µm, within the silicon. Electrical heating elements that are embedded resistors in the silicon heat the channels, and the electronics cycles the mixture through three precisely predetermined temperature profiles to amplify the DNA sample. The system then pumps the amplified DNA into the biochip’s detection area using microfluidics. The detector contains DNA fragments attached to the surface probe. Inside the probe, matching DNA fragments within the sample attach themselves to the fragments on the electrodes while the DNA fragments without matching patterns fall away. The system achieves accuracy by temperature control and detects the DNA fragments by illuminating them with a laser and observing which electrodes fluoresce.
This approach also reduces the size of the equipment because the external circuitry driving the chip is very compact (20 x 20 x 20 cm box), making it suitable for point-of-care applications. DNA analysis chips are used to diagnose genetic diseases, perform drug discovery, test livestock and monitor water supplies. The ST chip is unusual in that it is based on silicon, rather than on the plastic and glass substrate normally used for DNA detection. The silicon will allow much greater levels of integration, and both amplification and detection are performed for complete sample-to-result analysis.
IBM is another big participant in BioMEMS and one of the earliest commercial entrants. Its DNA BioMEMS chip (Figure 5) operates on a totally different principle that shows the versatility of this field. A MEMS chip is produced with very thin silicon cantilevered beams radiating outward on all four sides of the chip. Defection of any of these beams is detected since stress changes the electrical resistance. Another version causes beams to resonate at a specific frequency and any DNA adsorption reduces this vibration and it can be interpreted quantitatively. Each beam is coated with a bio-agent that can be DNA or other material that will selectively interact with the sample under test. Absorption by specific beams is detected by the chip and interpreted by the system. Each of the 250 silicon beams can be used to detect a different bio-agent.
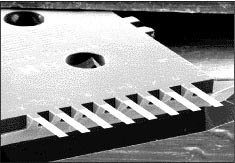 FIGURE 5: IBM’s BioMEMS chip, showing close up of beams.
FIGURE 5: IBM’s BioMEMS chip, showing close up of beams. |
Genome sequencing. Researchers at Whitehead Institute are testing a new MEMS-based gene sequencing system. Its BioMEMS 768 Sequencer can sequence the entire human genome in only one year, processing up to seven million DNA “letters” a day, about seven times faster than anything in use. Scientists began working on the project in 1999 with a National Human Genome Research Institute grant. The technology eventually will help scientists quickly determine the exact genetic sequence of the DNA of many different organisms, and could lead to faster forensic analysis of DNA gathered in criminal cases.
The core of the BioMEMS machine is a large chip etched with tiny microchannels or “lanes.” The number in the name is derived from the fact that it tests half, or 384 lanes, of DNA at a time. Each lane can accommodate progressively longer strands of DNA: about 850 bases (the nucleic acids found in DNA, abbreviated by the letters A, C, T or G), compared to the current 550 bases per lane. It takes about 45 minutes to read the DNA from one of the BioMEMS’ 768 lanes. The machine has two chips; one is prepared as the other is sequenced, so that the machine is running at all times. Savings come from low maintenance, speed and using smaller amounts of reagents. The BioMEMS system uses a DNA loading process that will eventually need only 1% as much DNA sample.
Other efforts to develop BioMEMS devices for clinical diagnostics have led to commercializing several related, but different, technologies used in massively high-throughput genomic screening assays. One of these lab-on-a-chip platforms is the LabChip product by Caliper Life Sciences and the GeneChip micro-array system by Affymetrix. Both systems offer the advantages of performing assays in microfluidic environments and reduce reagent usage, have faster assay times and can have the ability to perform reactions that are impossible in the macro world.
Commercial BioMEMS-based platforms have disposable cartridges and the lab-on-a-chip devices can handle microliter, nanoliter or even picoliter volumes. Systems contain a network of interconnected individual building blocks. The ability to design, test and fabricate these building blocks that include fluidic valves, channels, pumps and mixers that can process larger sample volumes is crucial to developing BioMEMS devices. BioMEMS platforms must include totally automated sample preparation of raw patient samples. This type of sample preparation has been lacking in lab-on-a-chip devices and target purification, extraction, capture and volume reduction have been handled “off chip.” If the manufacturer decides to include sample processing in the supporting instrument, then the instrument must remain compact amenable to point-of-care settings. Size of a complete unit is still a challenge that will require almost all devices to be micro- or even nanoscale.
The Micro Total Analysis System (µ-TAS) is a BioMEMS-based concept being developed at the Technical University of Denmark (DTU) north of Copenhagen in the city of Lyngby. The µ-TAS can perform functions on reagents and samples such as transportation, extraction, purification, mixing, separation, chromatography and detection. It can bring innovation to the chemical and environmental areas, but especially the biomedical field. Micro concave grating is used for the wave division demultiplexing of fiber communication with 81 channels. This optical micro-concave-grating was fabricated with advanced MEMS silicon etching techniques. This is a long-term project.
MEMS Augmentation
Hearing aids. Starkey Labs is using the Nanotech Chip for hearing aids from NVE. The chip uses a GMR sensor to detect the use of telephones and adjust the hearing aid accordingly. The sensors are based on a chip that uses electron spin rather than charge to store information and can be classified as nanoelectronic devices. The automatic switching frees a wearer of any need to switch manually. The sensor, one-third the size of the switch’s coil, is built from nanoscale layers of magnetic thin films just a few atomic layers thick. Tiny MEMS microphones are also now commercially available from several companies that can be used in hearing aids.
We can expect to see hearing implants in the future, however. A MEMS-based ear implant has been designed by the University of Michigan and looks like a cochlea. This appears to be the first hearing chip with integrated electronics and it uses micromachined cantilevered beams for sound interpretation. Beams of different lengths are used to distinguish frequencies. A piezoelectric vibrator will interact with the ear. A commercial version is still years away.
Sight aids. MEMS-based retinal implants research is being carried out by several laboratories including Sandia National Laboratories in conjunction with other DOE National Laboratories, the Doheny Eye Institute and Second Sight Corp. They are developing technologies to enable a conforming high-density electrode array for use in an implantable artificial retina. The project goal is to provide some level of useful vision to patients with diseases like macular degeneration and retinitis pigmentosa. Several implant designs are being developed in the U.S. and abroad, design challenges are significant. Second-Sight and the Doheny Eye Institute are performing clinical studies with a low-resolution implant of their own design. BioMEMS offers features beneficial to an artificial implant and truly unique to this technology. The present development effort will use external and internal devices as shown in the diagram (Figure 6).
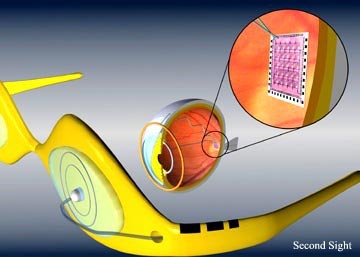 FIGURE 6: Second sight retinal implant.
FIGURE 6: Second sight retinal implant. |
Kidney. Can MEMS kidney chips be fabricated? The kidney is the number one organ needed for replacement with 35,000 Americans requiring kidney dialysis at a cost of $25 billion per year. There are 15,000 kidney transplants in the U.S. every year with 58,000 waiting. But should the artificial kidney be organic, an inorganic structure or a biological hybrid? Semiconductor processes using MEMS techniques can now make 3-D structures, so why not make a micro-filter or perhaps even a nano-filter? The artificial kidney system could end up being an entire wafer or an array of chips to meet the capacity needs.
Most developers think that, for now, that the artificial kidney needs to be outside of the body; in the future, it will be a transplant inside. The artificial device faces a challenge since the kidney filters 180 liters per day of blood. Draper Labs has a long history of MEMS development including fluidics. They are doing filter scale-up using microfluidic devices. Each layer has a micro-channel etched into a chip and scale-up just involves adding more layers using MEMS fabrication techniques. Massachusetts General Hospital is also doing work headed by Joseph P. Vacanti. He is using pattern silicon wafers as the master mold and making copies from plastic. The next step is to grow real cells on the plastic. The ultimate goal is a fully organic kidney, but an inorganic pump and filter system could be an interim step. Work is also going on at Brown University by Michael J. Lysaght, director for the center of biomedical engineering.
Heart. CardioMEMS, a member of Georgia Tech’s Advanced Technology Development Center (ATDC), is pioneering a BioMEMS sensor to monitor heart patients. They have combined wireless technology with BioMEMS to provide doctors with more information while making testing less invasive for patients. The FDA approved the unit under investigational device exemption (IDE) to enable the company to begin clinical trials in the U.S. for its EndoSensor. The EndoSensor measures blood pressure in people who have an abdominal aortic aneurysm, a weakening in the lower aorta. This condition ranks as the 13th leading cause of death in the U.S. If the aneurysm ruptures, the victim can bleed to death within minutes.
Doctors can treat an aneurysm with a stent graft, a slender fabric tube placed inside the bulging artery to brace it and relieve pressure by creating a channel for blood flow. Still, the stent can fail, resulting in leakage of blood into the aneurysm, which can cause the aneurysm to burst. For this reason, lifelong monitoring is required. The EndoSensor can be implanted to measure pressure in an aneurism being treated by a stent graft.
CardioMEMS’ biocompatible sensor, implanted along with the stent, monitors the stent more effectively than CT scans (Figure 7). It is also cheaper and more convenient. During checkups, patients do not even need to remove clothes. The physician merely waves an electronic wand in front of the patient’s chest. Radio-frequency waves activate the EndoSensor, which takes pressure measurements and then relays the information to an external receiver and monitor. Approximately 100 patients in four countries (the U.S., Canada, Argentina and Brazil) have received sensors. Data will be submitted from the trials to the FDA and the EndoSensor could start selling in 2005.
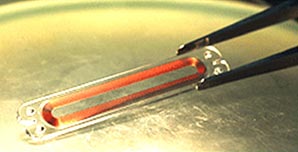 FIGURE 7: CardioMEMS pressure sensor.
FIGURE 7: CardioMEMS pressure sensor. |
CardioMEMS has also been advancing its HeartSensor, a wireless device that measures intracardiac pressure in patients with congestive heart failure. Similar to the EndoSensor, the HeartSensor is inserted through a catheter in a non-surgical procedure. Patients receive monitoring electronics to take home that are used to conduct daily pressure readings. The data are then transferred over a phone line to the patients’ physicians. HeartSensor enables doctors to monitor patients more closely and adjust medications as they see the disease progressing. The sensor detects a change in the body before any external symptoms are manifested and thus serves as an early warning system. Early prototypes were manufactured at Georgia Tech, but CardioMEMS has signed an agreement with an established MEMS foundry to produce the EndoSensor. The company has already demonstrated sensors that can be produced in large quantities at an affordable cost. Doctors can monitor the information from the chip in the office or remotely from patients’ homes.
Medtronic has developed the BioMEMS-based CareLink Monitor for defibrillator patients who can hold a monitor over their body to pick up the chip’s signals. That information is fed into a PDA or other device and then called in to the doctor by the patient or transmitted via the Internet to the physician over telephone lines. An implant is shown in Figure 8.
 FIGURE 8: Medtronic BioMEMS monitor.
FIGURE 8: Medtronic BioMEMS monitor. |
MEMS could also help improve pacemakers and defibrillators. The basic function of pacemakers has changed little in 20 years. All pacemakers include a computer, some memory and a telemetry link; additional sensors might provide more information to the user’s doctor, such as blood oxygen measurements, pH level or other information. Patients with pacemakers tend to be subject to other diseases as well, and a group of implanted sensors could provide early warnings about the patient’s health. Maybe someday they will be implanted in healthy people.
Heart tissue production. How about a new artificial heart? MIT is working in this area. It has come up with a living bandage concept. Heart cells are grown and subjected to poultices of electrical current so that they will react like the heart muscles do. The tissue made of the cells can be crafted upon to the heart where the muscle is weakened. It will beat in rhythm with the heart muscle since the heart sends out electrical pulses.
MEMS in medicine has already made an impact. The relatively new BioMEMS market was already at $215 million in 2001 and is projected to grow at more than 20% a year to $550 million in 2006 and finally top $1 billion by 2011, according to In-Stat-MDR and others. The demand could support a faster growth rate that is typically delayed by extensive and lengthy testing and approval requirements.
BioMEMS-based medical devices are now found in emergency rooms, surgical suites, doctors’ offices and even in personal health care devices. So it is quite possible that many of us have already been helped by this emerging technology. And if you have not yet had the encounter, the increasing application of BioMEMS in medicine means that you will have that experience in the future and it could save your life. Sensors are an important entry product class that has been able to leverage non-medical MEMS sensor technology. Both internal and external sensors, such as blood pressure monitors, are being deployed and enhanced. Lab-on-a-chip for point-of-care diagnostics and life science research is another very active area. MEMS technology is targeting a number of health care-related applications, including real-time circulatory system monitoring, glucose testing, pacemakers and heart monitoring, nerve and muscle stimulation and biotechnology analytical systems. Sensory aids and replacement devices are in testing and rudimentary vision restoration appears to be close. The most diversified and well-suited opportunity for MEMS in medicine will likely continue to be sensors, however. The next step is incorporation into surgical instrumentation. Other MEMS devices, particularly needles, probes and lab-on-a-chip, are also on the verge of very rapid growth. Needles/probes condition monitoring will bring novel means of drug delivery that will become an important category starting with diabetes treatment but expanding to other drug-treatable diseases as more knowledge is gained.
The Future
BioMEMS combines micro-mechanics, silicon chip semiconductor electronics and the basic living molecular processes; future results and discoveries will be phenomenal and sometimes unexpected. Visionaries will blend molecular biology with computational systems at the atomic scale to create bio-nano-electro-mechanical systems (BioNEMS) that could become a major factor in nanotechnology. But in the near term, many small biotech concerns and some major semiconductor makers will continue to commercialize products based on BioMEMS. In some areas, BioMEMS has improved existing medical technologies. But brand new devices in the medical sector will also be developed.
The BioMEMS impact on medicine and healthcare at the moment may be subtle but revolutionary. BioMEMS technology will enable us to advance medical diagnostics and therapies by reducing device size and cost while increasing capabilities. Future applications include miniaturized versions of drug delivery systems, transducers for ultrasound images and more implanted monitors with telemetry. Pressure and temperature sensors for minimally invasive surgery/followup are already here but will be reduced to easily implantable or wearable sizes. Researchers are investigating magnetic flow cell sorting for various diagnostic and therapeutic applications, such as rapid screening for cancer cells in blood or blood-forming stem-cell transplantation and in model cell systems of human peripheral lymphocytes, cultured cell lines and samples donated by patients, such as bone marrow. BioMEMS capabilities will increase and diversify in the future.
Analysis and monitoring will continue to be important areas for some time. While large and small companies have advanced DNA analysis, this is only the first step. Promising applications include DNA detectionm linking gene deficiencies to disease, drug design and patient monitoring. But there is a much larger field beyond DNA. The next step is proteomics, the study of how proteins are formed and how they operate. DNA has some mechanical abilities based on its lock-and-key method of bonding, but proteins are the physical building blocks and basic mechanical units of living organisms.
Although there are tremendous opportunities for BioMEMS in the medical market, major challenges loom ahead. The two biggest hurdles are the requirement of FDA approval as well as a constrictive supply chain that can be a daunting barrier to entry. These factors increase delays for this particular market.
Robotic surgery and augmentation is on the horizon. Microchips will be placed inside the body, such as on heart tissue for repair or inside of blood vessels for monitoring. The chips could contain cells that can generate specific types of tissue, such as muscles, organs, blood and others. Chips could also contain chemicals that would stimulate the growth of blood vessels, or medication that is slowly released into the body at specific points and under particular demands. The use of BioMEMS will move heart disease treatment to the next level and this has the potential to let physicians assess the benefit of their work right in the operating room instead of waiting for symptoms. A surgeon could also place a slow-release anesthetic in the wound at the end of the surgical procedure eliminating the need for traditional post-operation pain relief. A painkiller released slowly inside the body would prevent the pain impulses from reaching the brain so a patient would never feel the pain.
Preventive medicine will also be enhanced to enable us to diagnose the earliest abnormalities and plan more suitable and successful strategies. A chip might relay to physicians information on potentially cancerous tissues. BioMEMS chips equipped with sensors could detect mutated genes or dangerous levels of hormones, and enable doctors to determine which tissues to treat long before the situation goes critical. The day may come when MEMS-enabled micro-robots, or even “nanobots,” travel through the body to clear arteries and make repairs. These guided, or perhaps self-guided, self-propelled devices could be injected into the body to perform life-saving tasks or go on patrols to look for problems and do routine body maintenance. MEMS has already delivered tweezers, scalpels, injectors, cell-splitters, all kinds of sensors and several kinds of propulsion devices including turbines. In the future, your doctor may advise to take two MEMS and get some rest.
Ken Gilleo, Ph.D., is with ET-Trends LLC (et-trends.com); ken@et-trends.com




