Present and Future Solder Technologies
How new powders, activator chemistries and epoxy fluxes are evolving for production use.
History shows that the electronics assembly industry is always up for a good challenge. This was proved with the successful move from through-hole to SMT assembly, the elimination of CFCs from the cleaning process and implementation of Pb-free, to name just a few. Now, the industry is arguably at one of its biggest – er, smallest – challenges to date: extreme miniaturization. Although device footprint reduction has been an ongoing process over the past two decades, recent developments are some of the most exigent to date. Although designing much smaller packages presents its own unique set of hurdles (a topic for another article), the ability to incorporate these microscopic components into a high-volume, high-reliability production environment is at issue for assembly specialists.
Placing 0201s and 0.4 mm CSPs in a lab environment is one thing; achieving this feat reliably in high-volume manufacturing is quite another. A plethora of process variables are impacted by this reality, none likely as complex as the soldering process. Not only must solder materials accommodate much tighter pitches and smaller geometries, they also must maintain all the previously established requirements for modern manufacturing, including Pb-free capability, compatibility with higher reflow temperatures, humidity resistance, wide process windows and much more.
These new conditions pressure tried-and-true rules for solder materials such as stencil aspect ratios and surface-area-to-volume requirements. Here, we describe several developments on the solder materials front – from new powders to activator chemistries to epoxy flux technologies – to meet miniaturization trends.
As use of ultra fine-pitch devices grows and industry moves from 0201s to 01005s and from 0.4 mm CSPs to 0.3 mm CSPs, prevailing Type 3 solder pastes will no longer be sufficient to address smaller deposit volume requirements. Simply moving from Type 3 to Type 4, however, will not necessarily deliver the desired result either. Type 4 materials must be optimized for miniaturization demands.
In this instance, optimizing means tightly controlling not only the particle size, but the distribution of those particles within the material. While current industry standards tend to be a bit unclear as to allowable particle size in the upper end of the range, J-STD-006A (Table 1) is fairly liberal with the distribution range of particle sizes. But, recent testing has suggested a tighter distribution range and a smaller upper limit particle size may prevent some problems down the line.
Current work has focused on not only condensing the distribution and size range of the Type 4 particles, but also on producing the powder in such a way that the integrity of the surface finish is maintained, as this is also essential to lowering oxidation risk. The smaller particles of Type 4 materials make for a higher surface-area-to-volume ratio, which, in turn, introduces more opportunity for oxidation. Left uncontrolled, oxidation can lead to a variety of performance issues, including non-coalescence, poor wetting, or graping (more on that later), to name just a few. New powder production technology has delivered consistent, smooth surfaces, even on powder spheres less than 35 µm in diameter.
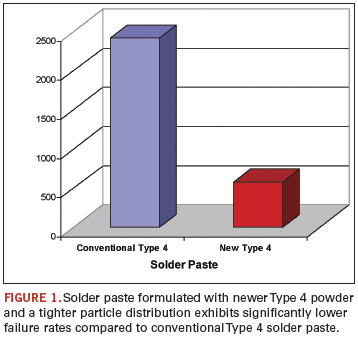
What’s more, by tightening the particle size distribution, the paste release from the stencil is much more complete. Larger particles can easily become trapped in the miniaturized apertures, leading to insufficients and down-the-line defects. By significantly reducing the upper and lower limits on the particle size in newer generation Type 4 materials, high-speed printing through 80 µm-thick stencils with 150 µm apertures becomes a much more robust process (Figure 1).
Pb-Free Solder Advances
Powder technology not only is critical in the move to much finer dimensions, but the overall capability of the paste and, specifically, the flux system is key. As 0201 integration has increased in production environments – particularly in the handheld sector – demands on smaller paste deposits have caused new process issues to emerge.
One such problem is graping. This phenomenon, which is partially coalesced solder that resembles a cluster of grapes, is directly attributable to extreme miniaturization (Figure 2). The cause of graping is easily understood, but not easily remedied without proper solder materials. With much smaller solder paste volumes, the solder particle surface-area-to-flux ratio is pushed to a point at which flux exhaustion occurs, the relative level of surface oxidation increases, and graping is the result. 
Flux’s function within the solder paste is to permit the formation of a solder joint by eliminating oxides that are present on metal surfaces – including the spheres within the paste. In addition, flux should protect the paste particles during reflow so as to prevent re-oxidation. As miniaturization requirements dictate the use of much smaller particle sizes (i.e., Type 4 and, in some cases, Type 5), the total metal surface of the solder increases and, therefore, demands more activity. Most powder oxidation occurs on the particles that are on the surface of the deposit. This puts more demands on the flux as the relative amount of solder surface is increased. Surface oxides generally melt at a higher temperature, and with older-generation formulations, the flux cannot overcome this condition.
By incorporating novel materials development technology, however, there are several ways to alleviate graping. As mentioned, smooth surface powders with a much tighter distribution range and upper/lower particle size limit greatly improve paste release from the stencil, deliver more even deposits, and provide a reduced metal surface and ideal deposit surface-area-to-volume ratio.
Next-generation solder paste flux formulations have proved that by providing sufficient activity and re-oxidation mitigation capabilities, graping can be resolved literally as it is occurring. Figure 3 illustrates this result, as traditional solder materials are compared to newer materials that have been optimized for miniaturization processes.
It also is important to note that while altering the flux and powder to accommodate for new process conditions, materials must also maintain their reliability requirements, as well as surface insulation resistance (SIR) and electrochemical migration (ECM) performance.
Heterogeneous Component Placement
Another obstacle presented by extremely miniaturized components is the dilemma about how to place the large and small components most efficiently. While new solder pastes are capable on both large- and small-volume deposits, stencil technologies are often the limiting factor. Designing a stencil capable of printing the large and small deposits in a single sweep is nearly impossible. A second print is out of the question, so the solution becomes dip fluxing.
Traditional dip fluxes certainly deliver the activity required to promote robust solder joint formation; the problem is how to then protect those joints. Capillary flow underfills will work only if there is a gap large enough to permit sufficient flow and coverage. Because this is a relatively large “if” considering newer component geometries, an alternative methodology should be considered.
The process is identical, but the material – an epoxy flux – is vastly different. Epoxy flux materials combine the solder joint formation action of a flux and the protection of an underfill into a single material. On a printed circuit board where one might need to place 0.3 mm CSPs, other very small types of area array devices or even flip-chip-on-board, epoxy flux is ideal for many reasons.
First, because the material combines the dual-functionality of a flux and an underfill, the secondary underfill dispense process can be eliminated. With epoxy flux, the solder joint is formed, and the epoxy surrounds and protects each interconnect during reflow. Second, even when capillary underfilling is an option, traditional underfill materials have exhibited problems such as component floating and voiding. A fluxing underfill, however, stays around or near the solder bumps to add an extra level of reliability without inducing floating or void formation.
For manufacturers faced with the heterogeneous – large and small – component conundrum, epoxy flux is an excellent option.
The Nano Future
An article on solder materials science would be sorely lacking without a discussion of what the future may hold. Temperature concerns and development of novel thermal management techniques are, with the advent of very small devices and Pb-free processes, more top of mind than ever. And, while significant progress has been made in relation to temperature control, applying knowledge from other markets may provide clues to soldering’s future.
As a case in point, transient liquid phase sintering (TLPS) is being evaluated as a thermal management solution. Used successfully in ceramic applications, the possibilities for TLPS in electronics manufacture are intriguing. TLPS processes rely on the combination of low-temperature melting alloy powders combined with higher-melting metal powders, which, when processed above the melting point of the lower temperature alloy, fuse to form a new intermetallic compound that will not re-melt at that same temperature but rather a much higher temperature. For electronics, this could conceivably mean that devices could be manufactured at significantly reduced temperatures and be able to withstand higher Pb-free processing temperatures with no risk of re-melt or damage. TLPS for electronics is very much in the infancy stage, of course, but many companies in the soldering industry are investigating its potential.
As technology marches on, so does materials innovation. In fact, in many cases, materials innovators are far ahead of the parade – developing materials for next-generation applications a good three to five years from becoming mainstream. These latest solder materials developments are further evidence of the ingenuity and expertise at the foundation of our industry. Solder solutions such as advanced powder technologies, more capable flux formulations, and dual-function materials such as epoxy fluxes are all enabling the smaller, faster, cheaper demands of the consumer to be fulfilled.
Solder materials science has indeed gotten small, but only because of big ideas and large innovation initiatives from leading materials scientists.
Neil Poole, Ph.D., is a senior applications chemist at Henkel Technologies (henkel.com); neil.poole@us.henkel.com. Brian Toleno, Ph.D., is senior applications chemist – assembly materials at Henkel.







