Electroless Plating of Palladium and Palladium Alloy Membranes
Considerations for incorporating fuel cells.
Cost, durability, size, weight, thermal and water management are major challenges to the commercialization of fuel cell technology in commercial and military applications. Many manufacturing issues need to be solved in order to bring fuel cells to the mass market for naval applications. Manufacturing and acquisition costs can be reduced significantly by reducing or eliminating the use of precious metal catalysts, such as platinum and rare earth metals, to reform fuels.
In light of these challenges, the Center for Advanced Mineral and Metallurgical Processing (CAMP) at Montana Tech is developing fuel cell manufacturing and materials technology for the Office of Naval Research ManTech Program to investigate electroless plating of palladium and palladium alloys on stainless steel substrates. This research includes laboratory work and an extensive literature search of electroless and other methods used to form palladium and palladium alloy membranes. This month, we summarize the findings.
Electroless plating is a chemical process that catalytically reduces metal ions in an aqueous solution and deposits that metal on a substrate without the use of electrical energy. The process is similar to electroplating, except no outside current is needed. A controlled pretreatment sequence and plating process are critical to produce good adhesion of the metal to the substrate.
In the electroless plating process, the driving force for the reduction of metal ions and their deposition is supplied by a chemical reducing agent in solution. In the case of palladium and palladium alloys, the reductant is typically hydrazine. This driving potential is essentially constant at all points of the component surface, provided solution agitation is sufficient to ensure a uniform concentration of metal ions and reducing agents. Electroless deposits, in theory, have a very uniform thickness all over the part. The electroless process has definite advantages when plating irregularly shaped objects, especially those that are difficult to plate by conventional means.
Hydrogen flux calculations. A unique property of palladium is that hydrogen gas diffuses through the metal. This characteristic is commonly used to produce very pure hydrogen using a palladium membrane. The flux of hydrogen gas through a palladium membrane is dependent on temperature, hydrogen partial pressure, and membrane thickness. From a metal cost and flux standpoint, it is desirable to make the palladium membrane as thin as possible. Therefore, instead of using pure palladium foil membranes, many researchers have investigated plating palladium onto porous metallic and non-metallic substrates.
A substantial amount of research has investigated hydrogen flux measurements through palladium and palladium alloy membranes. Hydrogen flux is the rate of hydrogen passing through the membrane per unit area. Flux is typically denoted by the formula: 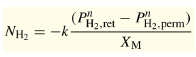
where:
NH2 = hydrogen flux in moles per second per sq. meter
K = permeability (moles per meter per sec. per pascal) PH2, ret , PH2, perm = the partial pressures of hydrogen on the retentate and permeate sides of the membrane, respectively (pascals)
XM = membrane thickness (meters)
According to Morreale,1 the exponent (n) in the above formula is equal to 0.5 if the rate-limiting step in the hydrogen transport mechanism is diffusion and that transport is unidirectional. However, the assumption that diffusion is the rate-limiting step may not be accurate. Morreale found that the optimum value for the exponent was greater than 0.5 in experiments as the hydrogen pressure on the retentate (upstream) side of the membrane increased. The optimum exponent value was determined to be 0.62 at retentate pressures up to 9,000 pascals and temperatures up to 1173 K. For this study, hydrogen flux values varied from 0.002 to 0.062 mol/s/m2, with greater values associated with higher pressure differential and higher temperatures. Experiments described in this study were conducted with relatively thick palladium membranes (XH ~ 1 mm).
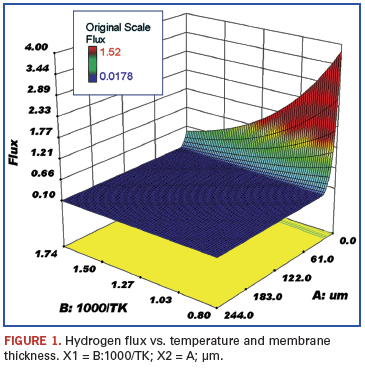
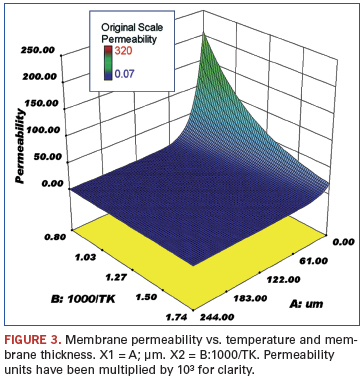
Data review and statistical analysis. Rothenberger in 2004 tabulated data from a large number of hydrogen purification studies.2 These data were imported into Stat-Ease statistical software and response curves created. The following figures show the effect of several variables on the trend for a particular response. These figures are for those sets of data where the plating was by electroless deposition and the permeate side pressure was atmospheric using a sweep gas.
The membranes were plated onto either aluminum oxide or porous stainless steel. The variables examined were palladium membrane thickness (µm), reciprocal temperature (1000/T K), and electroless versus other plating techniques. The responses were flux (mol/s/m2), permeance (mol H2/(m2•sec•Pan)•1012 (not shown), and permeability (mol H2/(m•sec•Pan)•105. The noted factors of 10 were used to make the axis numbers easy to read.
Figures 1 and 2 show the response of hydrogen flux with respect to changing temperature and palladium thickness. As the temperature is increased, the hydrogen flux goes up; flux is poor at lower temperatures, but is much greater the thinner the membrane and the higher the temperature. The flux, of course, at a membrane thickness of zero is only a mathematical projection. The lowest temperature shown is 300ºC, since palladium membranes must be operated above 300ºC to ensure a defect-free membrane. The usual operating temperature is in the range of 450º to 500ºC, and the desired membrane thickness is less than 5 µm. The problem of operating palladium membranes below 300ºC is that a mixture of body-centered cubic and face-centered cubic hydride solid solution phases is formed. Above 300ºC only a single solid solution phase exists. This problem is twofold; i.e., if the membrane is cycled above 300ºC and then back to ambient temperature several times, stresses are formed because of the different thermal expansion coefficients of the phases. The resulting stresses can cause defects in the membrane. One way to minimize the cycling problem is to treat the membrane at elevated temperature in a vacuum to remove dissolved hydrogen. In addition, the flux of hydrogen at ambient temperatures through palladium is very small, whereas it is much higher at elevated temperatures (300º to 600ºC).
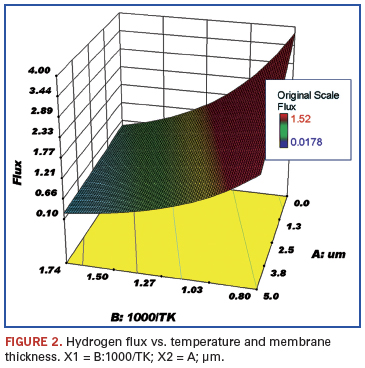
Figures 3 and 4 show the response of the membrane permeability to hydrogen with respect to changing temperature and palladium thickness. As expected, this follows the same trend as shown for hydrogen flux.
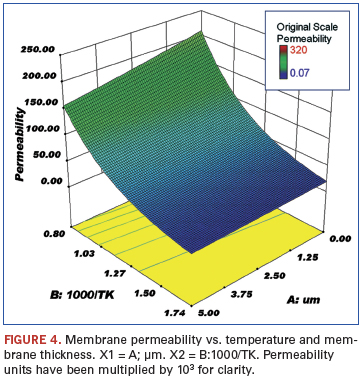
The fact that hydrogen flux increases with increasing temperature and decreasing palladium thickness is not surprising. The data appear to indicate room for improvement; higher temperatures and thinner membranes should result in even greater fluxes. However, there are practical limits to both these parameters in terms of material limitations. For example, it may be possible to deposit even thinner palladium membranes on substrates, but the integrity of these membranes will be compromised, especially when subjected to extreme temperature cycling. The same holds true for the higher temperatures needed for increased flux; many of the materials currently being used in purification systems are at their practical temperature limits.
References
1. B. D. Morreale, et al, “The Permeability of Hydrogen in Bulk Palladium at Elevated Temperatures and Pressures,” Journal of Membrane Science, 212.1-2, 2003, pp. 87-97.
2. K. Rothenberger, et al, “High Pressure Hydrogen Permeance of Porous Stainless Steel Coated with a Thin Palladium Film via Electroless Plating,” Journal of Membrane Science, 244.1-2, 2004, pp. 55-68.
ACI Technologies Inc. (aciusa.org) is the National Center of Excellence in Electronics Manufacturing, specializing in manufacturing services, IPC standards and manufacturing training, failure analysis and other analytical services. This column appears monthly.
Press Releases
- News Zollner Continues to Expand Capabilities in Thailand
- Keiron Printing Technologies Appoints PIT Equipment Services to Support Revolutionary LiFT Technology Adoption
- Surf-Tech Manufacturing Adds New ViTrox V510i AOI to Strengthen Advanced Packaging Inspection
- Altus Supports Cambertronics’ SMT Process Upgrade with Koh Young SPI







