2005 Articles
Surface Oxidation as a Tin Whisker Growth Mechanism
While water vapor accelerates whiskers, they also grow in ambient air.
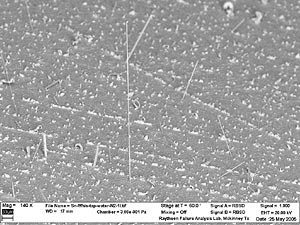
 FIGURE 1: Tin whiskers protruding from a tin-plated surface. |
A popular alternative to SnPb solders used for surface finish applications is pure tin. Pure tin plating can develop “whiskers,” outgrowths of small, wire-like protrusions from tin (Figure 1). These whiskers are electrically conductive and therefore can create the potential for short circuits in electronics. They can also break off and form conductive debris that could also cause shorts.1,2
The mechanisms of whisker formation are not understood, although several theories have been forwarded.2 Of these mechanisms, stress of the plated tin surface is widely believed to be a contributing factor. Oxide formation is associated with volumetric expansion of the surface layers of metal. This could conceivably cause a change in the mechanical stress in the surface. This possibility prompted the investigation of Sn-plated brass coupons exposed to oxidizing atmospheres, particularly humidity. Preliminary results are striking; moderate to rapid whisker growth occurred with most of the oxidizing atmospheres when compared to the control group.
Our first experiments involved placing Sn-coated brass coupons over cups containing deionized and tap water (McKinney, TX, municipal supply). After several weeks, whisker growth was noted on these coupons. Initially, we believed that whisker growth was more pronounced on the coupon suspended over tap water than DI water.
This preliminary observation led to an investigation of the tap water to identify possible contributing agents. There are several differences between tap and DI water – not the least of which is the presence of ionic salts. However, the presence of such materials seemed unreasonable as a contributing factor as they have limited volatility. Another, more likely compound present in tap water that may account for accelerated whisker growth is chlorine dioxide, a strong oxidant used in water purification. At this early stage, it was theorized that the tap water might present a more oxidizing environment than DI water, thus explaining our initial observations. As a result, an investigation was initiated to determine the role of water, both tap and DI, on whisker growth. Because oxidation was a suspect mechanism, experiments were also designed with the role of the ambient environment in mind (i.e., air, inert gas, etc.). Chemical pretreatment of coupons with common cleaners, which could contribute to whisker growth, was also investigated. As results of the following experiments show, moisture plays a significant role on whisker formation; however, it is not clear if DI or tap water preferentially accelerate whisker growth.
Methodology
Coupons used for the experiments were 1 x 3" brass strips with bright tin electroplating. One set of chemically treated coupons was rinsed in dichloromethane and another with a 10% aqueous solution of 60:20, ethanolamine:butoxyethanol, which was then rinsed with DI water to imitate a common inline board cleaner application. These two sets of coupons represent the extent of the chemical pretreatment investigation that was pursued. All other investigations were performed with “as received” coupons which were not manipulated in any way. Experiments were performed in most cases by placing two coupons over 12 oz. Styrofoam cups, filled to about 1/8" from the top of the cup with either DI or tap water (control cups were left empty). An additional experiment was carried out by vertically hanging single coupons over tap and DI water. In all cases, water was added biweekly to replace losses by evaporation.
To test environmental influence, one set of experiments was carried out in a nitrogen cabinet to create an “inert” atmosphere, another in room air and others in environments containing gaseous oxidizing materials.
More aggressive oxidizing environments were created by chemical reactions that liberated oxidizing gases. These were carried out in sealed desiccant chambers in which “as received” coupons were placed on top of the Styrofoam cups. Of these environments, only the results of an experiment regarding exposure of a coupon to nitrogen oxides formed upon reaction of nitric acid with metallic copper will be discussed. This experiment was accomplished by placing several pieces of copper at the bottom of one of the coffee cups, placing a coupon on the top of the cup and adding three drops of nitric acid to generate the gasses. The desiccant chamber was then sealed. Additional applications of nitric acid were performed at 24- and 48-hr. intervals, after which the coupon was not disturbed until examination one month later.
Mild Atmospheres
The first coupons observed growing whiskers (after six weeks) were those contained in the nitrogen cabinet suspended over water. Initially this seemed unlikely, as a nitrogen environment is more inert than room air. The control coupons suspended over an empty cup in the nitrogen cabinet did not grow similar whiskers. The accelerated growth of the coupons suspended over water was rationalized by the fact that the nitrogen chamber, as used, was actually venting nitrogen at a substantial rate leading to greatly accelerated water evaporation (these cups lost water to evaporation about four times faster than air samples). In essence, instead of creating an inert atmosphere experiment, we created an increased water evaporation experiment. These findings, as well as the initial experiments we carried out using only water, led us to conclude that oxidation of the tin surface as a result of exposure to water accelerates whisker formation. In fact, only the sides of the coupons facing the water showed accelerated growth; the top sides were not different than control room air samples.
For the experiments carried out in room air, similar results were noted, except whisker formation took longer before appreciable growth was noted (about three months in most cases). In these cases, water-facing sides of coupons grew whiskers at an accelerated rate while the air-facing sides and control samples did not. Also, these water-facing sides grew significantly more whiskers in areas directly over the water compared with the coupon areas that were outside the rim of the cups and not directly over the water. Additionally, the coupons vertically hung over water showed similar growth on both sides of the coupons (overall growth was more limited in these cases). The differences between DI and tap water, which were initially suspected, could not be proven by these experiments. The growth rate seemed somewhat random with some coupons suspended over DI water and others over tap water seemingly growing whiskers more rapidly. While some evidence suggests that chemical pretreatment may accelerate growth rates, it is impossible to verify from the data collected from these experiments. The following images are typical whiskers resulting from these experiments.
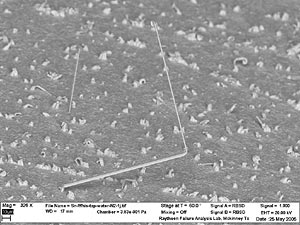
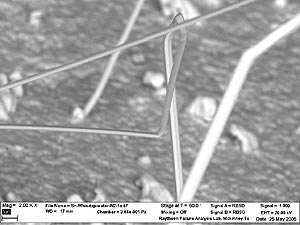
Figure 2 shows that while most whiskers are relatively thin, some variation in diameter is noted. Also, in most cases the whiskers grow unidirectional; but this is not always the rule, as noted in Figures 3 and 4. In fact, the whisker in Figure 4 is observed growing in five different directions along its length, forming a half-circle loop with an extension.
|
Extreme Atmospheres
The sample exposed to nitrogen oxides provided the most surprising observations. After one month, the coupon was removed and several dozen massively thick whiskers were noted. The number of whiskers was much lower than more typical examples, however, the diameters of these whiskers dwarfed those previously observed (Figure 5).
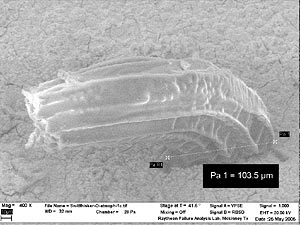 FIGURE 5: Tin whisker in O2 atmosphere. Diameter at base is about 100x typical. |
In this case rapid surface oxidation, resulting from exposure to the extreme environment, resulted in rapid stress formation that was relieved to a degree upon formation of these large whiskers. The rates of all processes occur more rapidly than the relatively slow water oxidation described in the previous section that leads to typical thin whisker formations.
Interestingly, after this coupon was exposed to room air for one day, extreme corrosion beyond that induced by the nitrogen oxides environment was noted. For example, oxidation of the whisker in Figure 5 was observed by SEM using backscatter mode (low atomic weight species, such as oxygen, appear dark in this mode, as in Figure 6).
 FIGURE 6: The dark color of the whisker indicates high levels of oxidation. |
These surprising results seemed to verify our assumptions that surface oxidation leading to stresses of the tin plate can accelerate whisker formation. More surprising and less easily rationalized is the fact that five days after the initial investigation revealed large whiskers, many more traditional sized whiskers were noticed on this same coupon – small whiskers grew less than one week after this coupon was exposed to ambient air. The morphology of the whiskers, however, is atypical. These were also observed to oxidize rapidly (Figure 7).
 FIGURE 7: Backscatter image indicating oxidation of whiskers formed after one week in air. |
Note that the ends of the whiskers in Figure 8 are rounded, leading to the appearance of tiny matchsticks. This is the first time we observed such morphology.
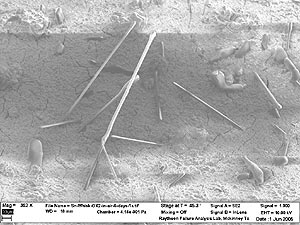 FIGURE 8: Whiskers grown in air in five days. |
Experiments carried out under aggressive oxidizing environments have been difficult to reproduce in a consistent fashion. Additional work is needed to characterize the conditions under which these features develop.
In conclusion, we believe that the examples presented reveal that oxidation of tin coatings accelerates whisker formation. This may be related to a change in the state of stress in the surface. The experiments performed with increased water evaporation grew whiskers more rapidly than samples exposed to water at normal evaporation rates. Moreover, both grew whiskers faster than coupons exposed simply to room air. Finally, in dramatic fashion, by exposing a coupon to an artificial oxidizing environment rapid and unusual whisker formation was observed. Further experimentation concerning oxidizing environments is ongoing. n
References
J.A Brusse, G. J. Ewell, J.P. Siplon, “Tin Whiskers: Attributes and Mitigation”, 22nd Capacitor and Resistor Technology Symposium Proceedings, March 2002, pp. 1-21.
W. John Wolfgong, Ph.D. is chemist, Bob Ogden is reliability engineer, Robert Champaign is failure analysis engineer and Barbara Waller is manager, Raytheon Failure Analysis Lab (raytheon.com); wolfgong@raytheon.com.
Press Releases
- PulseForge Makes Major Breakthrough Which Allows Flux-less Soldering in an Ambient Environment
- Panasonic Connect publishes case study on delivery of its surface mount technology (SMT) equipment to Dixon Technologies, one of India’s largest EMS companies
- CE3S and Desco Industries Announce May 2026 ANSI/ESD S20.20-2021 Certification Webinar Series
- Kurtz Ersa Goes Semiconductor: Expanding Competence in Microelectronics & Advanced Packaging