Current Issue
Field Performance of pH Neutral Cleaning Agents, Part II
Part two of a series on material compatibility and field cleaning performance of alternatives to alkaline cleaning agents.
Ed.: Part 1 looked at a pH neutral cleaning process using cleaning trials at an OEM customer on double-sided substrates assembled with Pb-free solder paste and flux using spray-in-air inline cleaner.
Customer B is an electronics manufacturing service provider to some of the world’s leading OEMs. The company specializes in PCBA and full product assembly, plastic molding, precision metal stamping, fabrication and finishing, and engineering services, with products ranging from simple consumer devices to complex, high-end commercial and industrial electro-mechanical products.
Customer B had an opportunity to qualify a new manufacturing process for a high-reliability electronic assembly for a medical application. The SMT process used no-clean solder paste and flux that required cleaning, for which the customer planned to use an aqueous-based inline cleaning process. In addition to sensitive materials on the substrate surface, the new process included use of anodized aluminum carriers to hold the substrates as they are conveyed through the cleaner. Thus, a cleaning agent with excellent material compatibility was required.
The customer collaborated with the company to develop the cleaning process for their qualification. Based on the process and material compatibility requirements, a pH neutral aqueous-based cleaning agent was selected.
Design of experiment. The customer substrate was designed as a panel, and each panel included 18 boards. Pallets and double-sided, populated, uncleaned substrates were provided to the company for the initial cleaning trials. In total, six panels and 12 boards were provided for the evaluation (TABLE 3). A spray-in-air inline cleaner was selected for the cleaning process.
Table 3. Test Boards
The main and speculum boards are part of the same assembly, and the name is used for reference only. Cleanliness was assessed as follows:
- Visual inspection per IPC-A-610E before and after cleaning.
- Ionic contamination analysis per IPC-TM-650.
- Ion chromatography analysis per IPC-TM-650; Method 2.3.28.
Boards and panels were utilized as detailed in TABLE 4. All panels were passed through the inline cleaner utilizing the anodized aluminum carriers. Carriers were inspected for surface integrity following the cleaning process.
Table 4. Test Boards Used by Customer B
With regard to the cleaning process, initial screening trials confirmed the pH neutral cleaning agent used at 10% concentration and 145°F wash temperature would be sufficient to clean the substrates. Optimized inline cleaner operating parameters are detailed in TABLE 5.
Table 5. Optimized Inline Cleaner Operating Parameters
Visual inspection results. Using the inline cleaner with the operating parameters detailed in Table 5, all boards and panels were cleaned in one pass. FIGURES 14 to 25 show before and after cleaning comparisons.

Figure 14. Before cleaning.
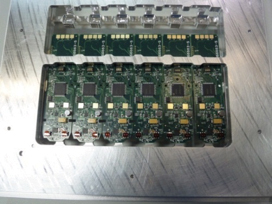
Figure 15. After cleaning.
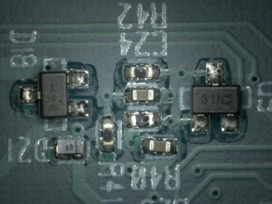
Figure 16. Before cleaning.
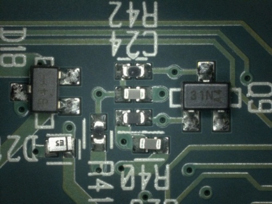
Figure 17. After cleaning.

Figure 18. Before cleaning.

Figure 19. After cleaning.

Figure 20. Before cleaning.
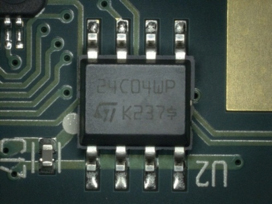
Figure 21. After cleaning.
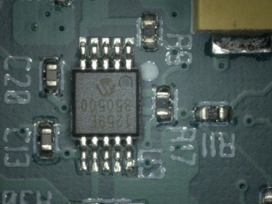
Figure 22. Before cleaning.
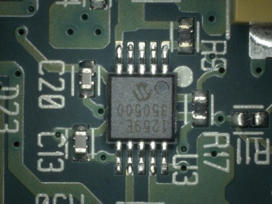
Figure 23. After cleaning.
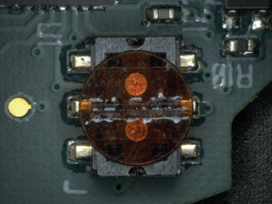
Figure 24. Before cleaning.
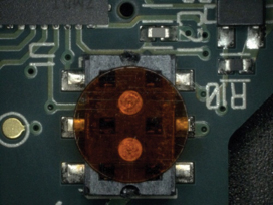
Figure 25. After cleaning.
Ionic contamination results. For the ionic contamination test procedure, four of the scrap boards were used. These were tested before and after the cleaning process. As expected, there is a significant difference in the results following the cleaning process. The Speculum board was also tested, but only following the cleaning process. This board passed as well (TABLE 6).
Table 6. Ionic Contamination Results
Ion chromatography results. One panel was cleaned and used for ion chromatography analysis. As detailed in TABLE 7, the board passed the ion chromatography test, as the ion species were below the maximum recommended contamination level.
Table 7. Ion Chromatography Results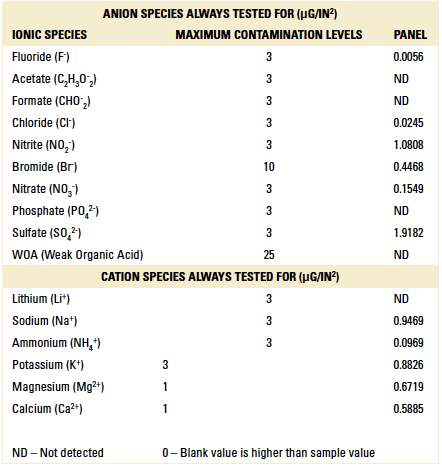
In conclusion, the pH neutral cleaning agent successfully cleaned all substrates, exhibited excellent material compatibility with all substrate components, as well as the anodized aluminum carriers, and required 10% concentration at 1.5 ft./min. belt speed and 145°F wash temperature, minimizing operating costs.
Case Study Review – Customer C. Customer C is an OEM designing and manufacturing high-reliability electronic control systems for the aerospace and energy industry. For one of Customer C’s applications, substrates were manufactured with water-soluble solder paste and flux and cleaned using a DI-water inline cleaner. Through visual inspection analysis, they confirmed residues were left untouched underneath the low standoff components, potentially leading to reliability issues. They wanted to explore the option of using a water-based, engineered cleaning agent to increase the cleanliness level for the given substrates and preferred to use a batch cleaner due to plant space constraints. However, if an aqueous-based cleaning agent is used, it must be compatible with their component materials, including anodized aluminum and olive drab cadmium. Also, given their geographic location, a closed-loop rinse cycle is mandatory, as rinse water cannot go directly to drain.
In collaboration with the company, a DoE was developed to explore the option of a chemically assisted cleaning process. Due to their requirements for material compatibility and a closed-loop rinse cycle, a pH neutral cleaning agent was selected.
Design of experiment. The DoE was divided into two parts with the following objectives:
Part 1: Utilizing populated company test vehicles, the pH neutral cleaning agent and a batch cleaner, assess and optimize process operating parameters to achieve the desired cleanliness level on the surface, as well as underneath components as measured by visual and ion chromatography analyses. Next, compare these results to those achieved with the customer’s DI-water cleaning system.
Part 2: Utilizing the equipment and optimized process parameters from Part 1, and the customer’s test boards, assess the cleanliness level achieved as measured by visual and ion chromatography analyses. Additionally, assess the effect of the cleaning agent on the sensitive materials.
Part 1: Methodology. Since the customer requirement included cleaning under low-standoff components, the company test vehicle was used. Eight boards were populated with chip capacitors (0402, 0603, 0805, SOT-23, 1206, 1210, 1812, and 1825) at the customer facility using the selected water-soluble paste and flux (FIGURE 26).
Figure 26. Test board.
Two of these boards were cleaned at the customer site using its current DI-water cleaning process. These boards were returned to the company technical center for cleanliness assessment, one each for undercomponent and ion chromatography assessment. The remaining six boards were also returned for cleaning trials using the pH neutral cleaning process and cleanliness assessment.
Based on screening trials using four of the test boards, optimized operating parameters were defined and are detailed in TABLE 8. Two additional boards were cleaned using these parameters, and both were assessed for surface cleanliness. The components were removed from one (1) board to enable undercomponent cleanliness assessment and the other used for ion chromatography analysis. FIGURES 27 to 34 show the undercomponent cleanliness comparison.
Table 8. Optimized Inline Cleaner Operating Parameters

Figure 27. Underneath 1825 component after cleaning using DI-water.
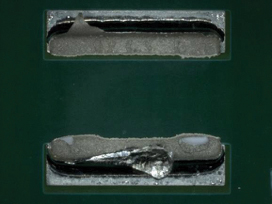
Figure 28. Underneath 1825 component after cleaning using pH neutral.

Figure 29. Underneath 1812 component after cleaning using DI-water.

Figure 30. Underneath 1812 component after cleaning using pH neutral.

Figure 31. Underneath 0402 component after cleaning using DI-water.

Figure 32. Underneath 0402 component after cleaning using pH neutral.
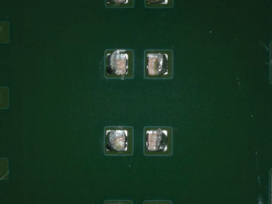
Figure 33. Underneath 0603 component after cleaning using DI-water.

Figure 34. Underneath 0603 component after cleaning using pH neutral.
Table 9. Visual Inspection Results
Part 1: Ion chromatography results. Two boards were used for ion chromatography analysis, one cleaned with DI-water and another with the pH neutral cleaning agent. Results are detailed in TABLE 10. Both boards passed the ion chromatography test, as the ion species were below allowable maximum contamination levels. However, the total ionic contamination present on the board cleaned with the pH neutral cleaning agent was less than what was present on the DI-water-cleaned board. Additionally, the undercomponent assessment of the DI-water-cleaned board confirmed untouched flux residue remained under several components, providing the opportunity for field reliability issues under use (Figures 27 to 29).
Table 10. Ion Chromatography Results
Part 2: Methodology. Having confirmed low-standoff components can be fully cleaned on the surface, as well as underneath the components, the customer was provided with five test boards for process assessment (FIGURE 35).Using the same batch equipment and optimized parameters as in Part 1, the boards were cleaned and assessed for cleanliness using visual and ion chromatography assessment. The customer boards were double-sided, thereby increasing the cleaning challenge. Thus, it was determined to increase the wash temperature to 150°F, compared to 140°F used in the Part 1 trials. All other batch cleaner operating parameters remained the same.
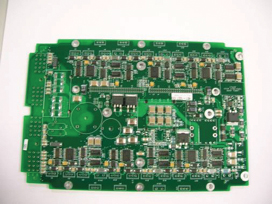
Figure 35.
Part 2: Visual inspection results. All five boards were cleaned using the optimized cleaning process. Surfaces were fully cleaned on all boards. Of the five boards, one was used for ion chromatography analysis. Of the four remaining boards, components were removed, enabling undercomponent cleanliness assessment. Of the 100 components inspected, only four components from two boards had slight residue remaining, while all others were fully cleaned underneath. FIGURES 36 to 43 show the undercomponent post-cleaning results. The sample board passed the ion chromatography test. The ion species were below allowable maximum contamination levels.
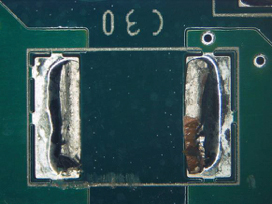
Figure 36. Underneath C30.
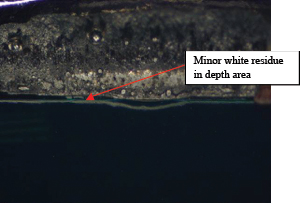
Figure 37. Underneath C82.
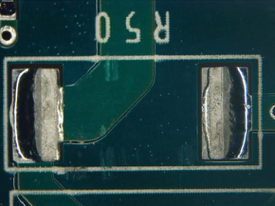
Figure 38. Underneath R50.
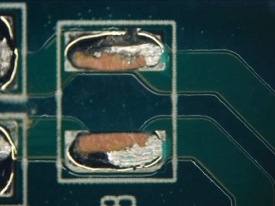
Figure 39. Underneath C108.
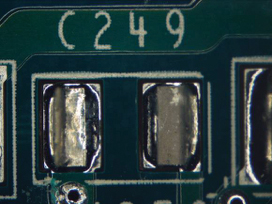
Figure 40. Underneath C249.
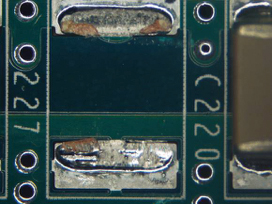
Figure 41. Underneath C227.
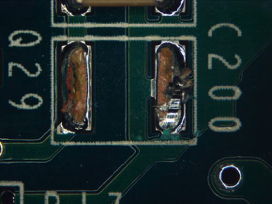
Figure 42. Underneath C200.
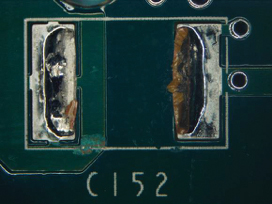
Figure 43. Underneath C200.
Overall, Customer C found the pH neutral cleaning agent provided excellent cleaning results on the surface and underneath the components with an optimized batch cleaning system. It exhibited excellent material compatibility with anodized aluminum and olive drab cadmium material. The process required 10% concentration at 1.5 ft./min. belt speed and 150°F wash temperature, minimizing operating costs.
Overall Conclusions
This study presented three case studies, each with a different paste/flux vehicle and material compatibility constraints. Based on the results, pH neutral cleaning agents can meet the stringent cleaning requirements expected, even with burned-in flux residues from multiple heat cycles, while exhibiting excellent material compatibility, with lower wash concentrations compared to alkaline alternatives.
Ed.: This article was originally published as “pH neutral Cleaning Agents – Market Expectation and Field Performance” in the IPC Apex Expo Proceedings and is republished here with the authors’ permission. Part 1 was published last month.
, is application technology manager, , is senior applications engineer, and is application engineer, at Zestron Americas (zestronusa.com); umut.tosun@zestronusa.com. is a former application engineer at Zestron.
Press Releases
- PulseForge Makes Major Breakthrough Which Allows Flux-less Soldering in an Ambient Environment
- Panasonic Connect publishes case study on delivery of its surface mount technology (SMT) equipment to Dixon Technologies, one of India’s largest EMS companies
- CE3S and Desco Industries Announce May 2026 ANSI/ESD S20.20-2021 Certification Webinar Series
- Kurtz Ersa Goes Semiconductor: Expanding Competence in Microelectronics & Advanced Packaging







